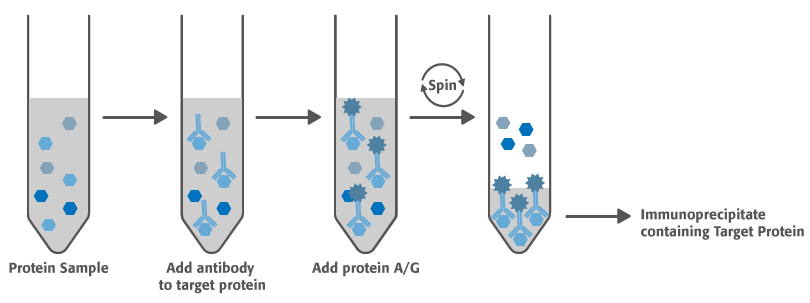
The combined procedures of immunoprecipitation and SDS-PAGE can be a powerful tool to assess the amount and size of an antibody-reactive antigen present in a complex protein mixture. The basic protocol uses a primary antibody followed by a secondary antibody-agarose conjugate to immunoprecipitate the antigen.
Reagents Required
- TBS: Use 10XTBS, pH 7.5 (1.0M Tris HCl, 1.5M NaCl). Dilute appropriate volume to 1X with dH2O. Store at RT up to one month.
- Lysis Buffer: TBS, 1.0% of an appropriate detergent (i.e. Triton X-100), 1mg/ml BSA and an appropriate proteinase inhibitor.
- Dilution buffer: Same as lysis buffer without proteinase inhibitor.
- Agarose conjugates for lysate pre-treatment. Pre-adsorb lysate to remove non-specific binding to primary and secondary antibodies. Use agarose normal IgG from the same species as the primary antibody and the host secondary antibody. Prepare washed slurry at 1:1 using dilution buffer.
Primary Antibody
- Control for primary antibody: For polyclonal antiserum, use nonimmune serum from the same species. For monoclonal antibodies, use the same isotype and purity.
- Agarose conjugates for Immunoprecipitation: Use agarose secondary antibody conjugate against the same species as the primary antibody. Prepare washed slurry at 1:1 using dilution buffer.
- Tris Buffer: Prepare 0.05M Tris buffer, pH 6.8.
- 2XSDS-PAGE Sample Buffer.
- 2-Mercaptoethanol
Procedure
- Prepare lysate by incubating 5 x 10e7 cells in lysis buffer for 30 to 60 min on ice.
- Vortex lysate and centrifuge for 10 min at 250 x g to remove nuclei. Retain supernate.
- Clarify supernate by centrifugation for 30 min at 100,000 x g or microcentrifuge for 30 min at 10,000 x g.
- Pretreat lysate to remove nonspecific protein binding by adding agarose conjugates. Use 10ul of control agarose per 200ul lysate. Shake for 1 hour at 4°C. Centrifuge at 200 x g. Save supernatant.
- Add 200ul of pretreated lysate containing antigen to each of two microfuge tubes. Bring volume to 1ml with dilution buffer.
- Add primary antibody to one tube. For polyclonal antiserum or ascites fluid use 0.5-5.0ul. For tissue culture supernatant use 10-100ul. To the second tube add an equivalent volume of control for primary antibody. Incubate on ice for 1 hour.
- For immunoprecipitation add 50ul of agarose conjugate per tube. Mix with gentle shaking for 1 hour at 4°C.
- Centrifuge tube 1 min at 200 x g or microcentrifuge for 5 seconds. Carefully remove the supernate with a pipette. Gently resuspend pellet in 1ml dilution buffer. Repeat wash. Follow with a wash in TBS and then a final wash in 0.5M Tris, pH 6.8.
- Centrifuge again as above. Add 20-50ul of sample buffer. Mix and heat for 5 min at 100°C. Microcentrifuge briefly and apply supernate directly to nonreducing SDS-PAGE. If reducing conditions are desired, transfer the supernate to a new tube and add 5% 2-mercaptoethanol. Mix and heat as above.
- Electrophorese protein mixture. Stain gel or immunoblot to visualize. Bands present will include polypeptides of antigen and antibodies used.